
Audit-ready SOP management without Enterprise bloat
Compliance document management system built for QA & Ops teams in food, biotech, and regulated manufacturing. Compliance without the enterprise bloat.
SOP tracking is a nightmare for regulated SMBs, a constant slog and thorn in the side of every QA manager
❌ Google Docs & SharePoint → Not CFR 11 compliant❌ Enterprise QMS tools (Veeva, MasterControl) → Expensive, slow, and overbuilt❌ Manual workflows → No audit trail, error-prone
SOprep makes compliance simple:
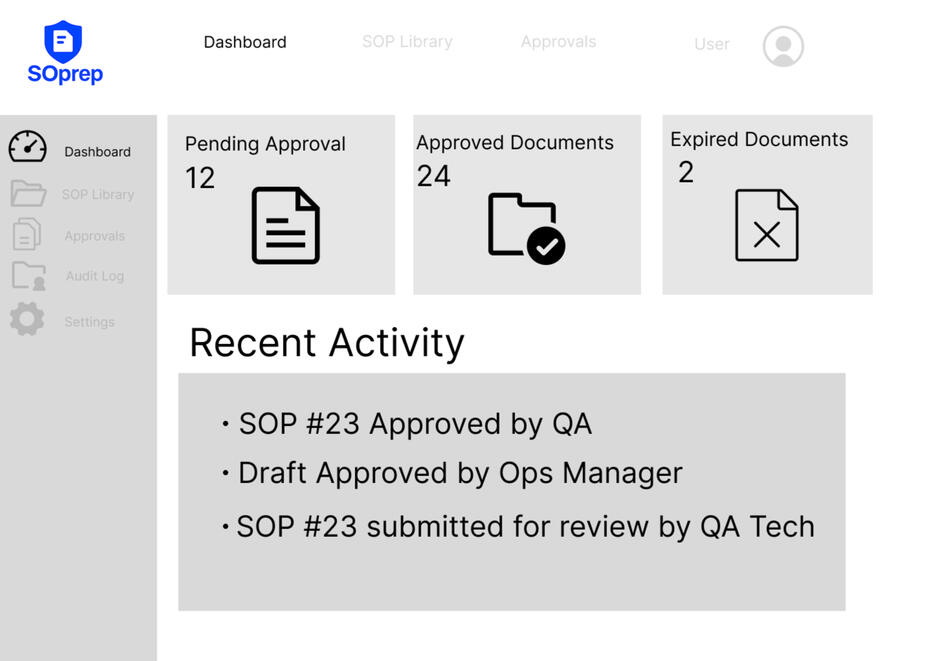
✅ Built-in version control✅ Secure electronic signatures✅ Automatic audit trails✅ Lightweight & affordable✅ Easy setup — no IT team required
Designed with CFR 11 in mind
✅ Audit logs for every change
✅ Role-based access controls
✅ Signature & approval workflows
✅ Permanent version history